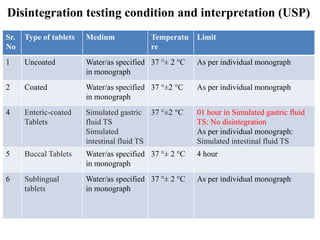
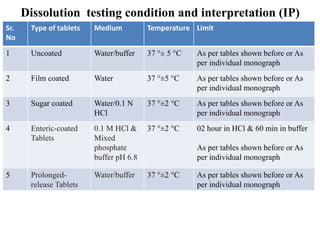
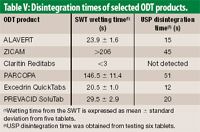
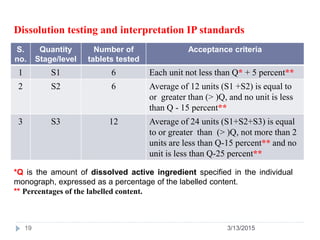
![PDF] Application of a Modified Disk for Testing Orally Disintegrating Tablets by USP | Semantic Scholar PDF] Application of a Modified Disk for Testing Orally Disintegrating Tablets by USP | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/0b9b1e1497cdca12af9f8044328c1614606d5c0e/3-Table2-1.png)
PDF] Application of a Modified Disk for Testing Orally Disintegrating Tablets by USP | Semantic Scholar
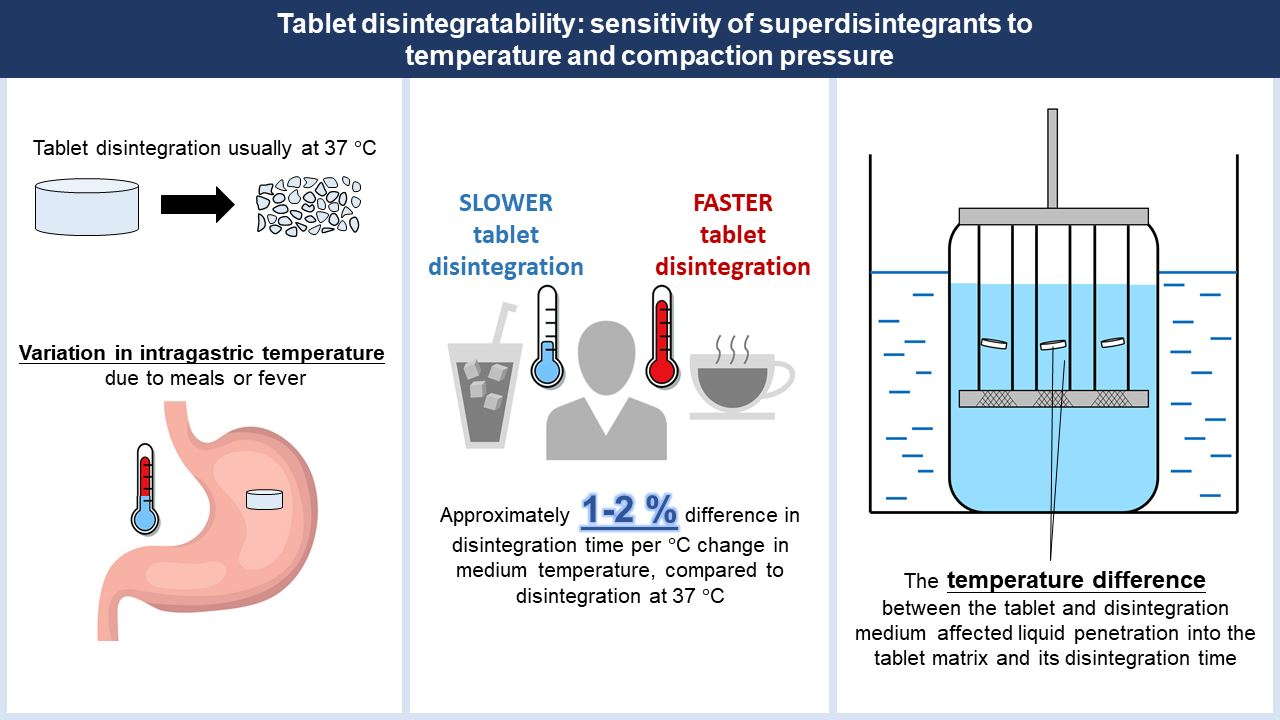
Pharmaceutics | Free Full-Text | Tablet Disintegratability: Sensitivity of Superdisintegrants to Temperature and Compaction Pressure
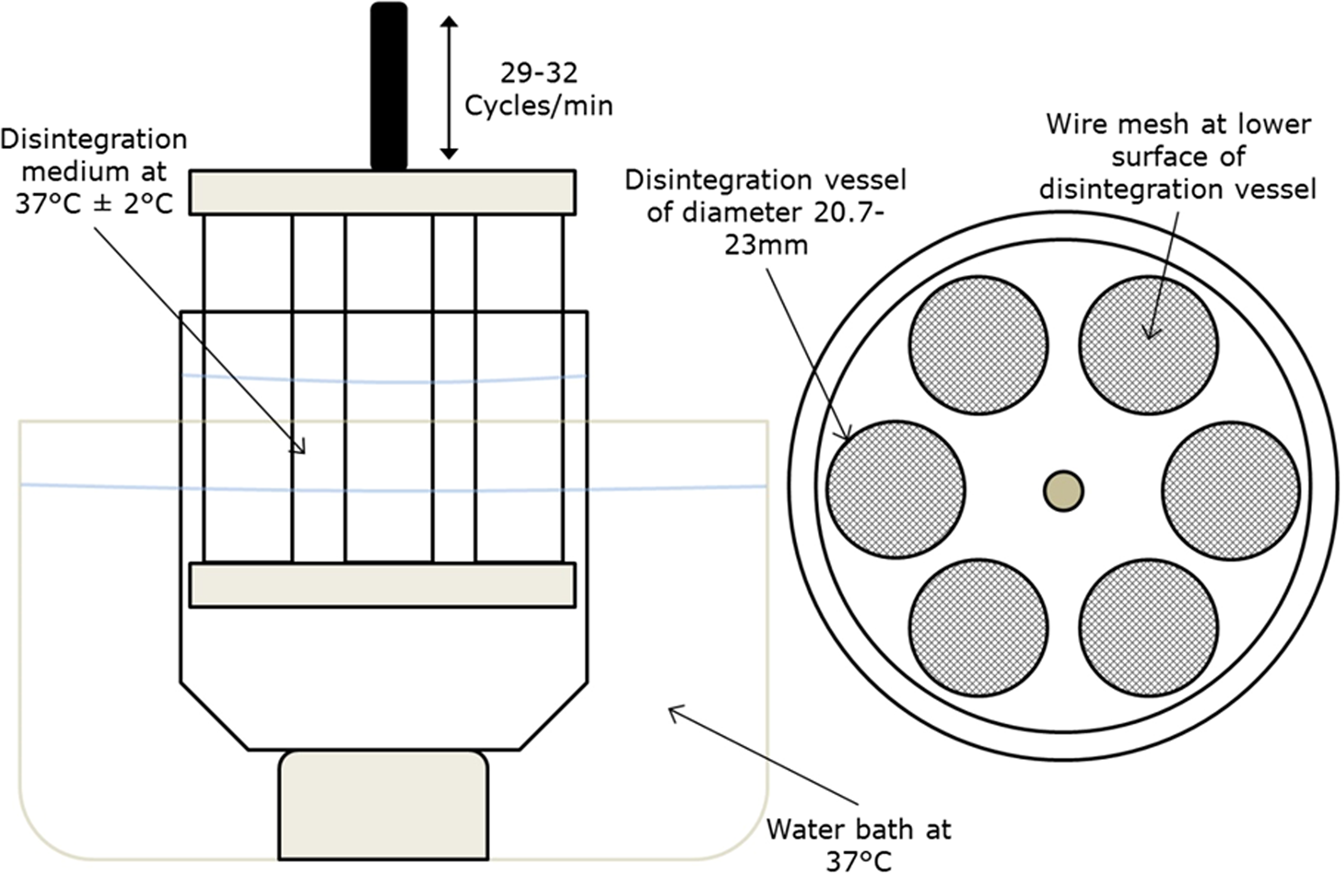
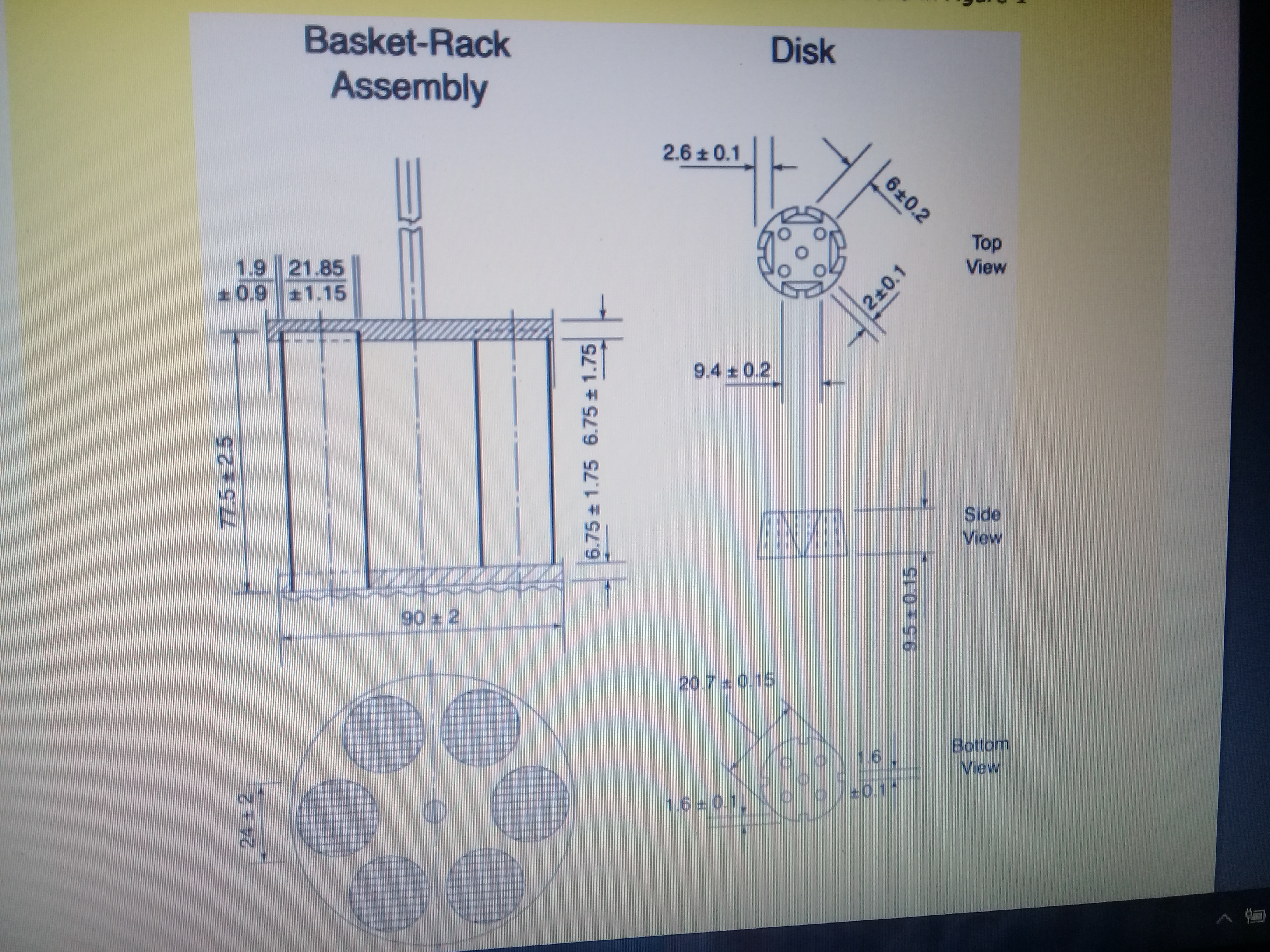
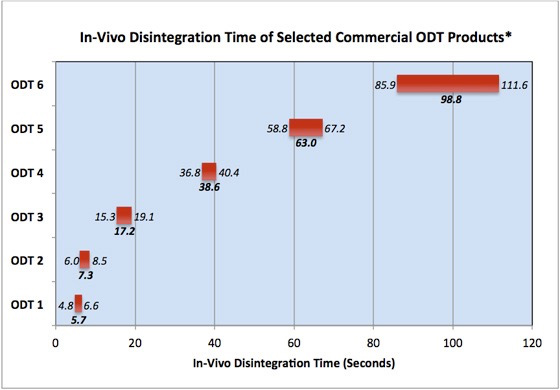
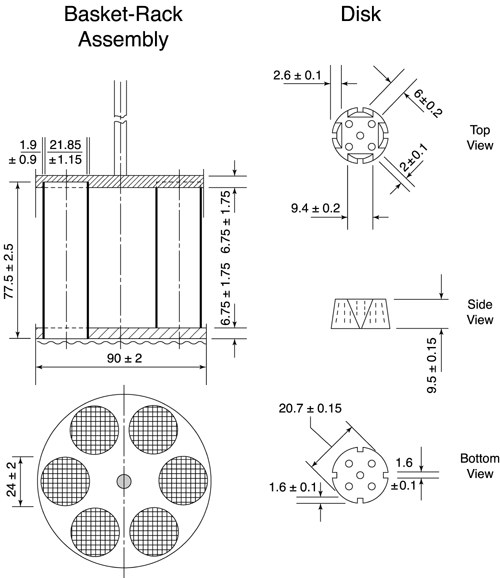
Conceptualisation, Development, Fabrication and In Vivo Validation of a Novel Disintegration Tester for Orally Disintegrating Tablets | Scientific Reports
18 Disintegration Test Interview Questions And Answers For Quality Control “ Pharmabeej | by Pharmabeej | Medium