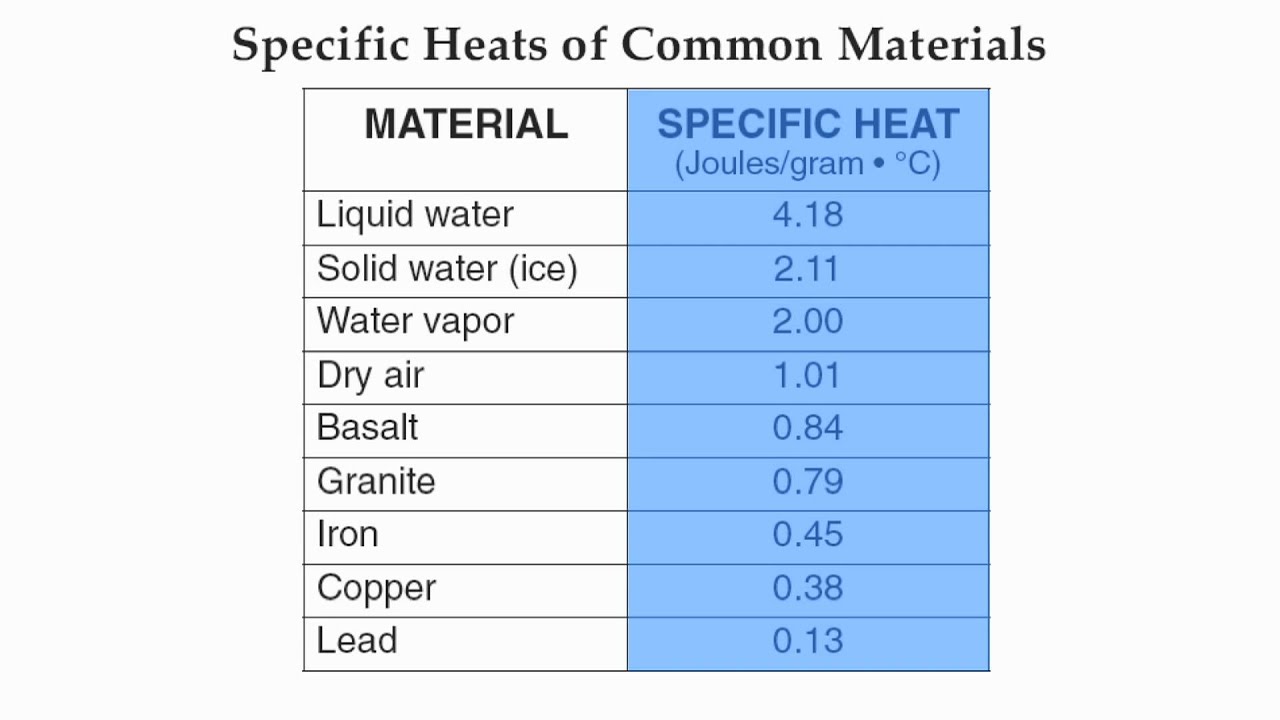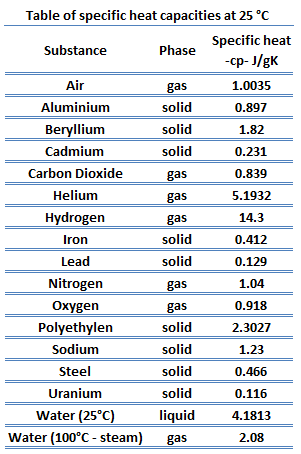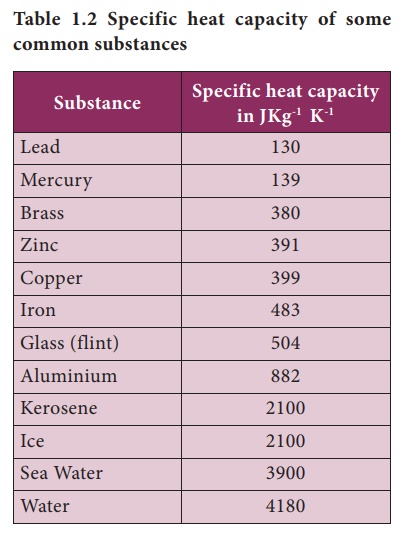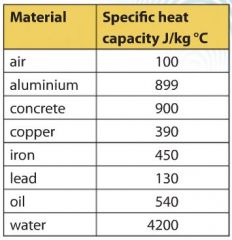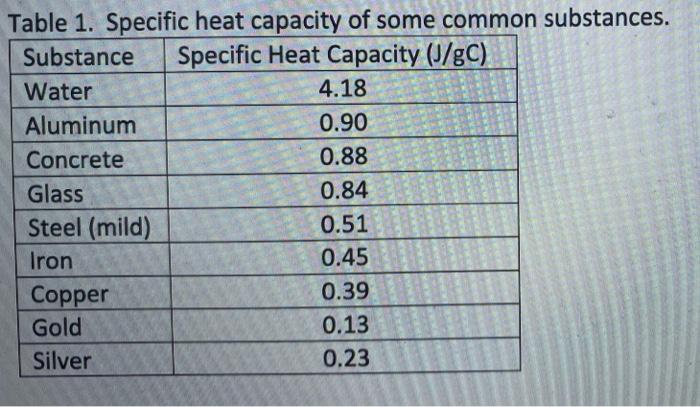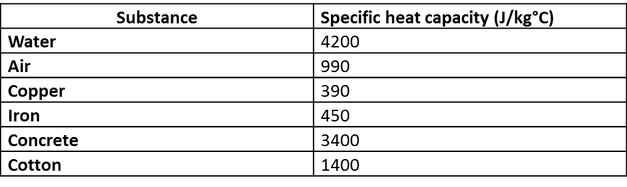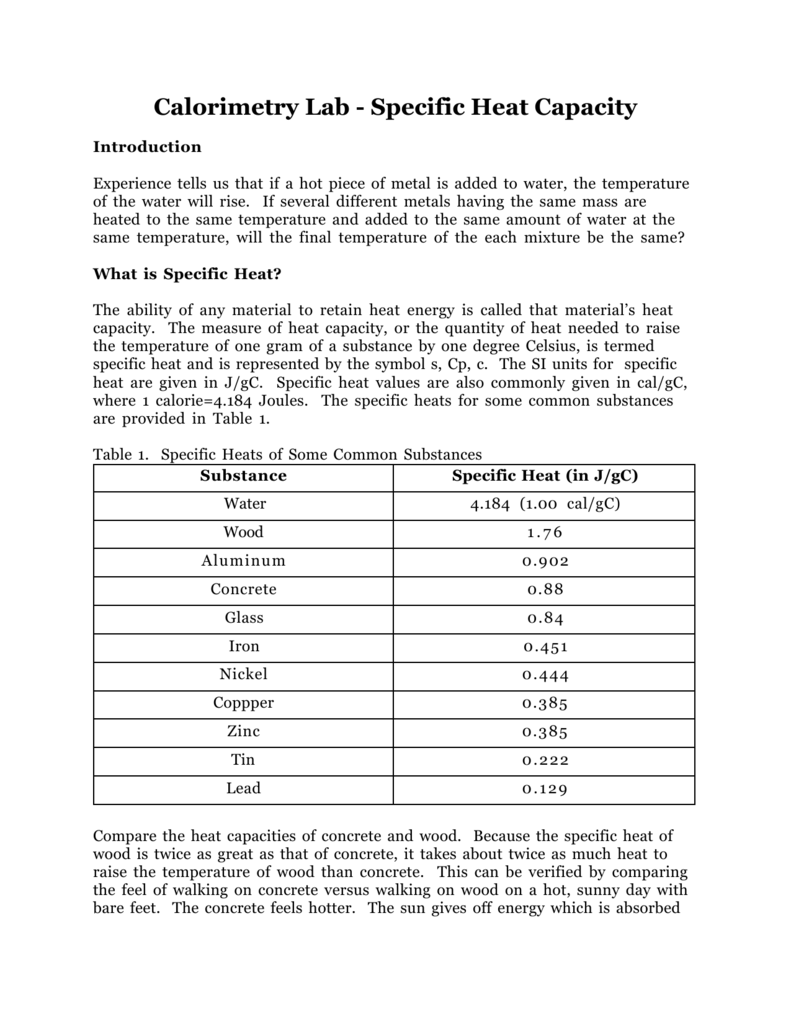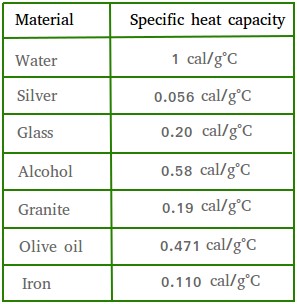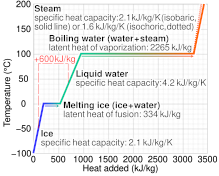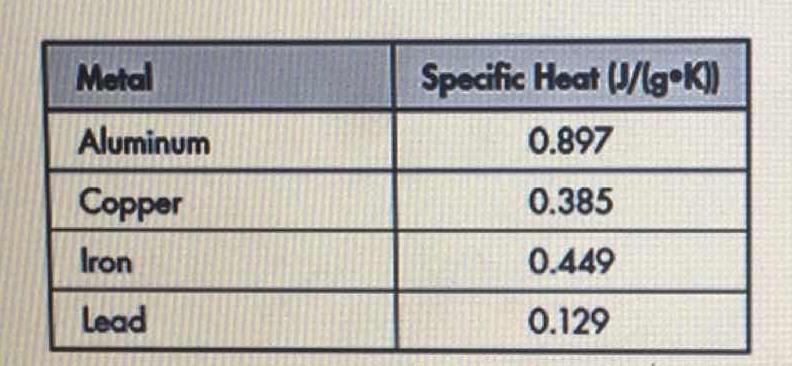
The table below shows the specific heats of several metals. The temperature of a 15-g sample of an unknown metal increases from 20.0 C to 30.0 C when it absorbs 67.5 J

Derived mean values of the specific heat of pure iron in comparison... | Download Scientific Diagram
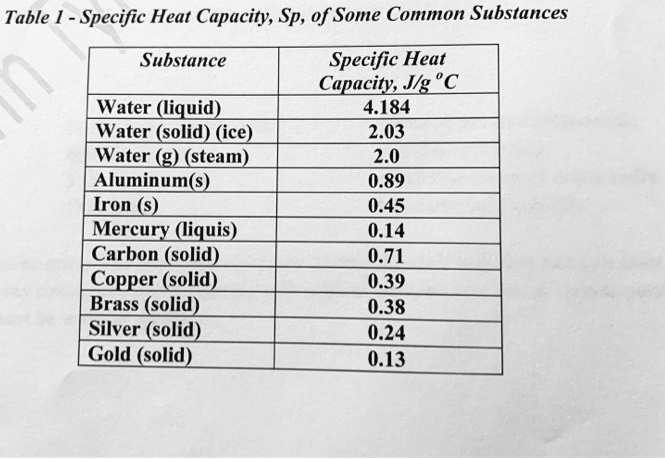
SOLVED: Table 1 Specific Heat Capacity, Sp, of Some Common Substances Substance Specific Heat Capacity, Jg 4.184 2.03 2.0 0.89 0.45 0.14 0.71 0.39 0.38 0.24 0.13 Water (liquid) Water (solid) (ice)
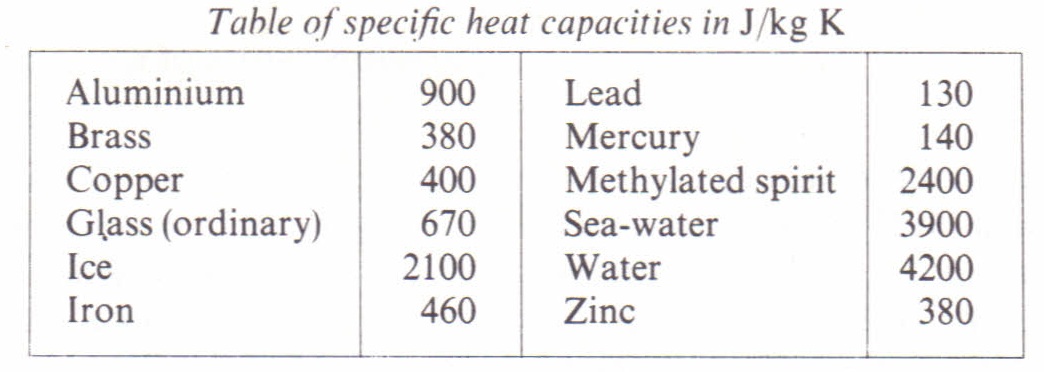
Specific heat capacity Physics Homework Help, Physics Assignments and Projects Help, Assignments Tutors online

Color online) Temperature-dependent specific heat capacities of (a)... | Download Scientific Diagram

Calculate the energy required to heat 790.0g of iron from −2.6°C to 14.9°C. Assume the specific heat - Brainly.com
A piece of iron of mass 2.0 kg has a heat capacity of 966 J K 1.Find:i heat energy needed to warm it by 15∘C,and ii its specific heat capacity in S.I.
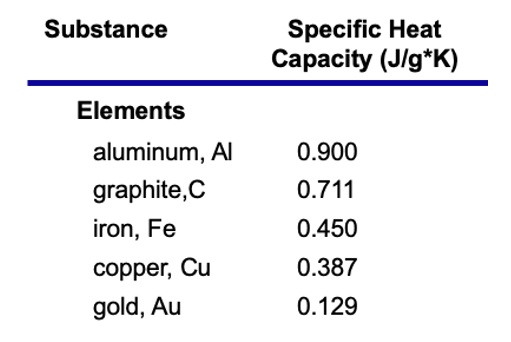
SOLVED: Substance Specific Heat Capacity (Jlg*K) Elements aluminum, Al graphite,C iron, Fe copper; Cu gold, Au 0.900 0.711 0.450 0.387 0.129